Ionic bonding results in compounds known as ionic or electrovalent compounds which are best exemplified by the compounds formed between nonmetals and the alkali and alkaline earth metals in ionic crystalline solids of this kind the electrostatic forces of attraction between opposite charges and repulsion between similar charges orient the ions in such a manner that every positive ion.
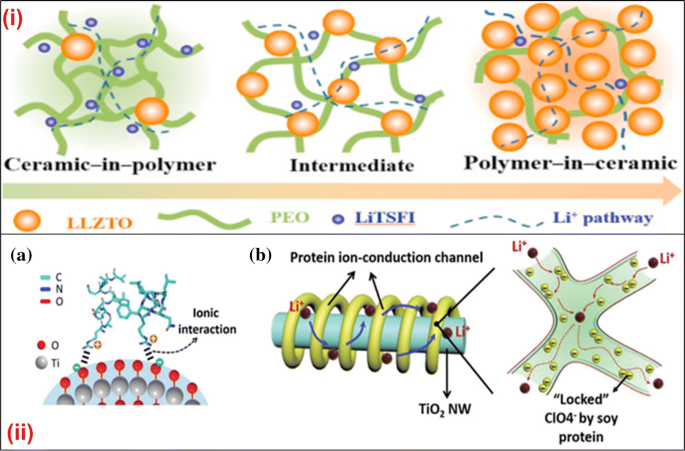
Charge carriers ionic ceramic.
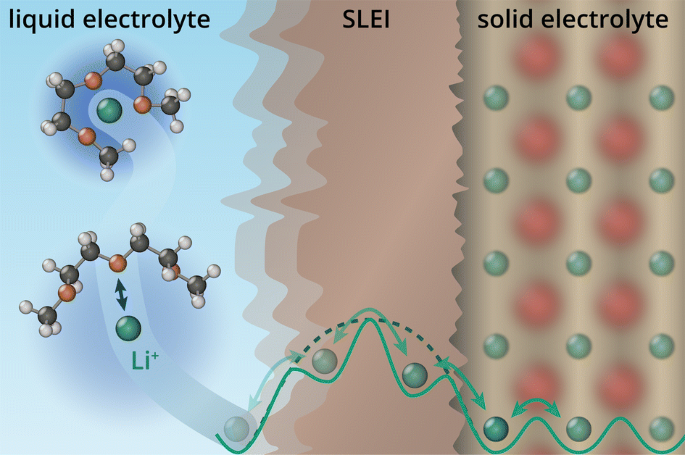
The mobility of the ions charge carriers is given by.
However in nonionic solids ionic conduction is extrinsic depending almost entirely on the nature and concentration of ionic impurities.
Although both types of bonds occur between atoms in ceramic materials in most of them particularly the oxides the ionic bond is predominant.
All of the above.
This is what constitutes an.
Metallic and the van der waals.
If the majority charge carriers are accumulated in the space charge regions a considerable con ductivity enhancement may result.
This occurs only in pure ionic crystals.
Ionic polarization is polarization caused by relative displacements between positive and negative ions in ionic crystals for example nacl.
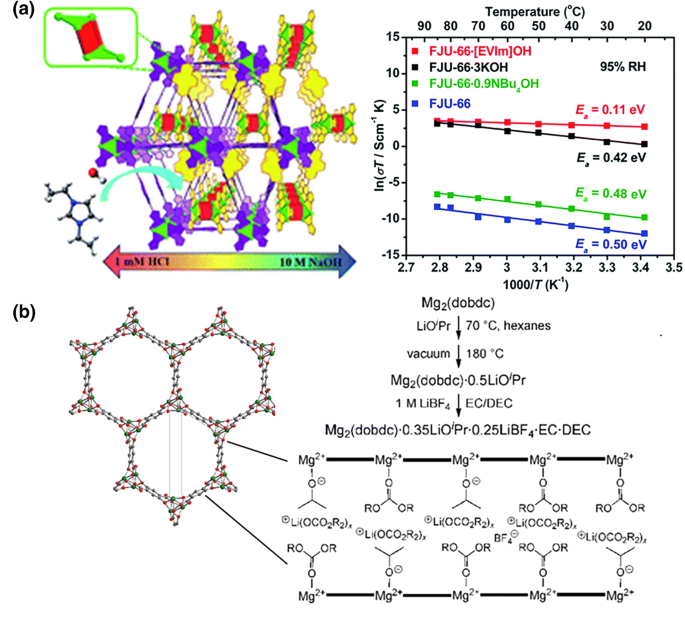
Significant space charge associated charge transfer resistance with similar typical double layer capacitances 1 the implication of space charge at metal oxide interfaces on the transport properties of ion conductors and interfacial mass storage has been qualitatively explored in a number of studies.
Given that a vacancy mechanism is observed in most solid ionic conductors at reduced temperature extrinsic domain ion trapping can lead to a major enhancement of the ionic conductivity.
If a crystal or molecule consists of atoms of more than one kind the distribution of charges around an atom in the crystal or molecule leans to positive or negative.
There are two other types of atomic bonds.
D is the diffusion coefficient.
You might also like.
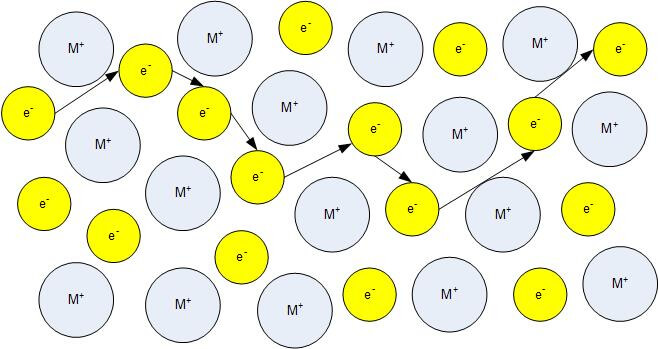
The charge carriers in ionic ceramics can be electrons holes anions and or cations.
In physics a charge carrier is a particle or quasiparticle that is free to move carrying an electric charge especially the particles that carry electric charges in electrical conductors examples are electrons ions and holes in a conducting medium an electric field can exert force on these free particles causing a net motion of the particles through the medium.
Where q is the electronic charge.
Ionic conduction it often occurs by movement of entire ions since the energy gap is too large for electrons to enter the conduction band.
In the first one the metal cations are surrounded by electrons that can move freely between atoms.
Hence obtaining interfacial configurations that promote the formation of oxygen vacancies is highly desi.