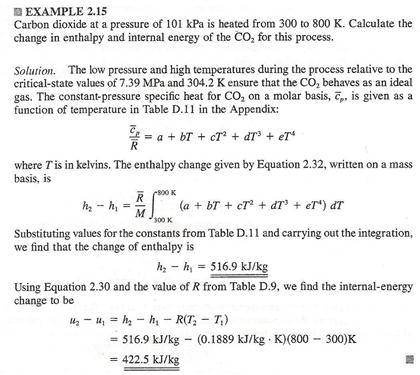
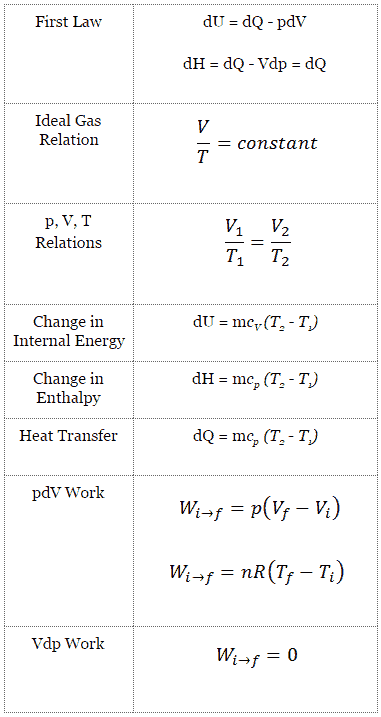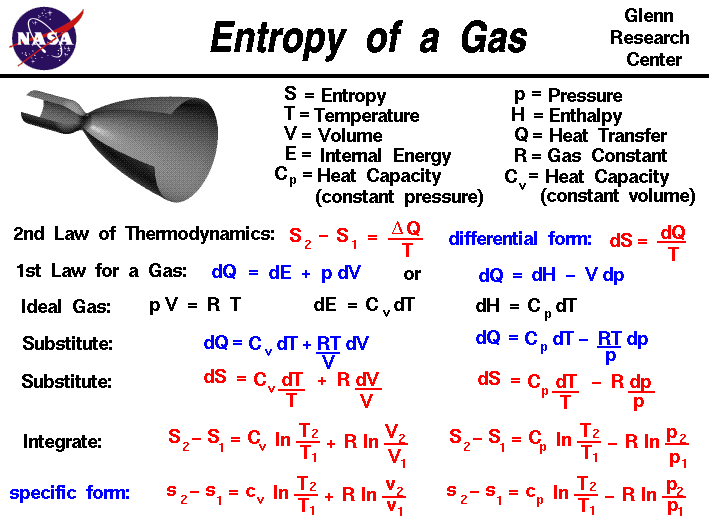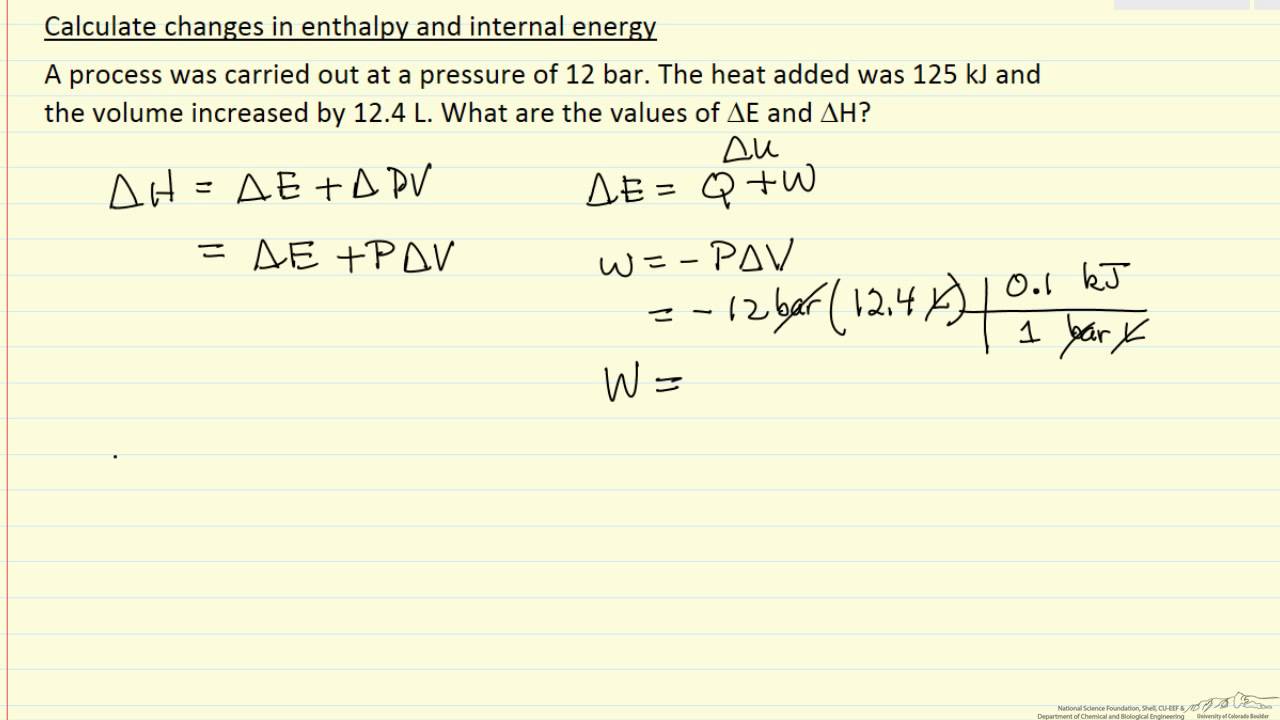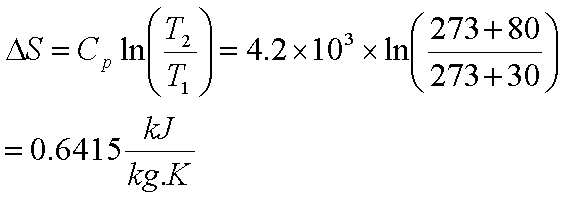H qp at constant pressure the relationship between the change in the internal energy of the system during a chemical reaction and the enthalpy of reaction can be summarized as follows.
Change in internal energy formula constant pressure.
δ p 0.
Where the subscripts v and p denote the variables held fixed.
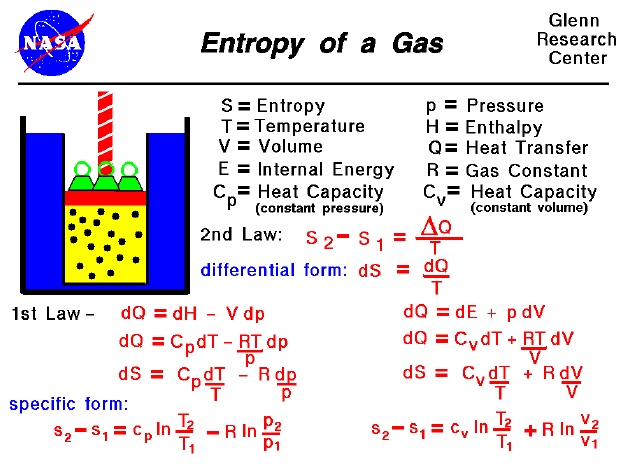
The first law of thermodynamics states that the change in internal energy u for a system is equal to the heat added to the system q minus the work done by the system w or in symbols.
An isobaric process is a thermodynamic process in which the pressure stays constant.
It is usually formulated by stating that the change in the internal energy of a closed system is equal to the amount of heat supplied to the system minus the amount of work done by the system on its surroundings.
U q w u q w.
It keeps account of the gains and losses of energy of the system that are due to changes in its internal state.
The heat given off or absorbed when a reaction is run at constant volume is equal to the change in the internal energy of the system.
The heat transferred to the system does work but also changes the internal energy of the system.
If you are working with an ideal gas mixture for which the internal energy of the reactants and products is not a function of pressure the change in internal energy in going from reactants to products at constant temperature and volume is the same as the change in internal energy in going from reactants to products at constant temperature and pressure.
Specific heat at constant volume and constant pressure.
When the volume of a system is constant changes in its internal energy can be calculated by substituting the ideal gas law into the equation for δu.
P is gas pressure v is volume is the number of moles r is the universal gas constant 8 3144 j ok mole and t is the absolute temperature.
The intensive properties c v and c p are defined for pure simple compressible substances as partial derivatives of the internal energy u t v and enthalpy h t p respectively.
The first law of thermodynamics the conservation of energy may be written in differential form as.
The change in the internal energy of a system is the sum of the heat transferred and the work done.
Specific heat is a property related to internal energy that is very important in thermodynamics.
The first law of thermodynamics is a version of the law of conservation of energy specialized for thermodynamic systems.
The internal energy of a thermodynamic system is a measure of the energy within it excluding the kinetic energy of motion of the system as a whole and the potential energy of the system as a whole due to external force fields.